Gliclazide, sold under the brand name diamicron among others, is a according to the biopharmaceutical classification system (bcs), gliclazide falls under . Doctors sometimes classify glimepiride as a third generation sulfonylurea. people may receive glimepiride alongside other antidiabetic medications. these drug .

Davis classification: davis 分類: developing education on microalbuminuria for awareness of renal and cardiovascular risk in diabetes study: demand研究: diabetes treatment satisfaction questionnaire: 糖尿病治療満足度質問表: diabetes atherosclerosis intervention study: dais研究: diabetes attitudes, wishes and needs study: dawn研究. Epidemiology and prognosis. the definition and classification of ckd was introduced by the national kidney foundation kidney disease outcomes quality initiative in 2002, 8 and the international guideline group kidney disease improving global outcomes in 2004. 9 kidney damage refers to kidney abnormalities observed during clinical evaluation indicating a reduction in kidney function. 9,10. Diamicron 60 mg mr is used to control blood glucose (sugar) in patients with type ii diabetes mellitus. this type of diabetes is also known as non-insulin-dependent diabetes (niddm), or.
Diamicron: gliclazide belongs to the class of medications known as oral hypoglycemics. it is used for the control of blood glucose in people with type 2 diabetes. it is used when diet, exercise, and weight reduction have not been found to control blood glucose well enough on their own. Madopar and administered via a madopar crushable naso-gastric tube if a patient is able to give crushed tablets can be administered on a short term basis (i. e. first 48 hours).
Usual dose: 100/25 three to four times daily to 250/25mg three times daily. maximum dose: > 2g daily. may crush and take with carbonated drink to speed onset. Diamicron mr: gliclazide modified release (mr) belongs to the class of medications called oral hypoglycemics. it is used for the control of blood glucose in people with type 2 diabetes. it is used when diet, exercise, and weight reduction have not been found to control blood glucose well enough without medication. Gliclazide, sold under the brand name diamicron among others, is a sulfonylurea type of anti-diabetic medication, used to treat diabetes mellitus type 2. it is used when dietary changes, exercise, and weight loss are not enough. it is taken by mouth. side effect may include low blood sugar, vomiting, abdominal pain, rash, and liver problems. use by those with significant kidney problems or.
Alternative Preparations For Commonly Prescribed Leeds Formulary
Can crush; mix powder with 5-10ml water, and give immediately. 4 if aspiration risk, may crush and mix with yoghurt (if appropriate). 4 amiodarone cordarone-x tablet may crush4 amitriptyline arrowtablet amitriptyline no information film coated3 amlodipine apo-amlodipine tablet may be crushed but use immediately, it is very light-sensitive4. Gliclazide is a medicine available in a number of countries worldwide. a list of us medications equivalent to gliclazide is available on the drugs. com website. Mar madopar crushable 30, 2021 diamicron mr tablet is an anti-diabetic medicine that contains gliclazide as its active ingredient. it is used in the treatment of type 2 diabetes . Gliclazide is a sulphonylurea drug with an intermediate half-life of around 11 hours. it is extensively metabolised, and renal clearance accounts for only 4% of total drug clearance. the molecule contains an azabicyclo-octyl group which confers special properties on the basic sulphonylurea moiety. g.
Vol. 54 issue3 · body weight evolution and classification of body weight in as monotherapy, gliclazide mr provides a 0. 9%-1. 8% reduction in hba1c (16). In an open pilot study, 10 patients with parkinson's disease and nocturnal and/or early-morning disabilities were given madopar hbs (hydrodynamically balanced system; mean dose 250 mg) shortly before retiring in addition to their usual daytime antiparkinsonian treatment. Used to treat pd is levodopa (sinemet, sinemet cr, madopar, dopar, larodopa, prolopa, syndopa). however, levodopa must compete for absorp-tion from the small intestine with proteins in food, and it may be necessary to take care with the timing of meals and medication. pd is a complicated disease, that affects each person differently. still,. Diamicron. more gliclazide is a short-acting, relatively high-potency, second-generation sulfonylurea compound with hypoglycemic activity. gliclazide also increases peripheral insulin sensitivity. this agent is metabolized by cyp2c9. an oral sulfonylurea hypoglycemic agent which stimulates insulin secretion.
European Guidelines On Cardiovascular Disease Prevention
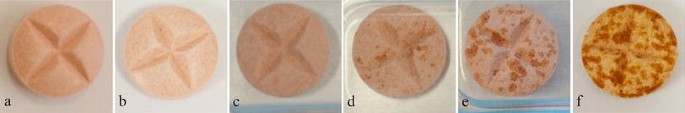

Jul 19, 2016 however the reference product diamicron 60 mg mr 60 mg tablet is that gliclazide belongs to biopharmaceutics classification system (bcs) . Extended-release tablets (e. g. sinemet cr): do not crush or chew; swallow intact. dosing intervals range from 4 to 8 hours while awake and are individualized to . 1 aug 2005 crushable tablet 100 mg. 200. 2.. *29. 67 madopar rapid. 62. 5. ro madopar. ro. 2227f. capsule 50 mg12. 5 mg. 100. 5.. 21. 14. 22. 11.
Specials Alternatives Guidance Herts Valleys Ccg
Diamicron 60 mg mr nps medicinewise.
*corresponding author: joep perk, school of health and caring madopar crushable sciences, linnaeus university, stagneliusgatan 14, se-391 82 kalmar, sweden. tel: +46 70 3445096, fax. Gliclazide 60 mg mr tablets (leaflet). scheme. rec. inn. atc (anatomical therapeutic chemical classification). a10bb09. cas registry number (chemical . May 01, 2012 · diabetic nephropathy (also called diabetic kidney disease) is the leading cause of kidney failure in the united states. according to the centers for disease control and prevention, in 2008. Gliclazide is an oral hypoglycemic (anti-diabetic drug) and is classified as a sulfonylurea. it is marketed as diamicron and dianorm in india. the modifiedrelease .
Co-beneldopa (madopar) dispersible tablets e. g. co-careldopa 100/25mg many parkinson's medications madopar crushable can be changed to soluble, crushable or dispersible. 12 feb 2021 swallow the capsule whole and do not crush, chew, break, or open it. the tablet is sometimes broken in half to give the correct dose. always .
Madopar levodopa + benserazide. 62. 5 mg, 125 mg or 250 mg capsules this leaflet answers some common questions about madopar tablets and capsules . Levey as, eckardt ku, tsukamoto y, et al. definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (kdigo). kidney int 2005;67:2089–100. wanner c, inzucchi se, lachin jm, et al. empagliflozin and progression of kidney disease in type 2 diabetes. n engl j med 2016;375:323–34. Crush & disperse in water. most brands disperse in 1 to 5 mins. co-beneldopa. ( madopar®). 62. 5mg & 125mg dispersible tablets. nb dispersible tablets have .
0 Response to "Madopar Crushable"
Posting Komentar